Inspired by last year’s theme of ‘Engaging patients for patient safety’ on World Patients Safety Day, one of our core mandates is ensuring better health care by championing patient safety and vigilance. Concerning this, we seek to create a sense of awareness amongst Kenyans to the extent that they can ensure the safety and effectiveness of the medication they consume. We endeavour to empower the patient to partner with the healthcare professionals in patient safety.
The Kenyan Pharmacy and Poisons Board (PPB) has developed an easy and practical way of reporting the harmful effects of drugs and poor-quality medication by phone – SMS USSD *271#. This action has been made possible by the Mobile Pharmacovigilance Electronic Reporting System (mPvERs) II, which PPB launched in March 2021. This innovative scheme allows Kenyans to report using their mobile phones in the comfort of their homes, making it easily accessible and convenient for all citizens. This method is fast, simple, inexpensive and easily accessible. In addition, it neither requires a smartphone nor internet connection. In this article, we highlight why reporting is necessary, how the USSD code works, and how it can enhance the quality of health care in Kenya.


An adverse drug reaction (ADR) refers to a negative unintended effect(s) that may result from the use of a particular drug or combination of drugs. Reporting the adverse effects of drugs and substandard medications enables the regulatory body in Kenya (The Pharmacy and Poisons Board) to detect, investigate and respond rapidly to potential issues. This promotes better quality pharmaceuticals in the market, thus ensuring the safety of patients. In this way, individuals become potential contributors to a safer, more effective healthcare system and, hence, also become part of the solution.
Below is a step-by-step guide on how the USSD code works:
- Dial *271# on your mobile phone.
- Get registered by following the prompts to provide general information about yourself and your whereabouts for first-time users.
- Once you have been registered, dial *271# again and follow the prompts to provide the necessary information on adverse drug reactions or poor-quality medication.
- Submit the report, after which you will receive a confirmation message.
Reporting via USSD *271# is essential in developing a solid pharmacovigilance system where the PPB can continuously monitor and evaluate medication safety. Sharing your experiences enables authorities to recognise patterns of possible threats and thus take adequate steps like recalls or market withdrawals. Your contribution is a step towards improving pharmaceutical product development and a safer healthcare system.
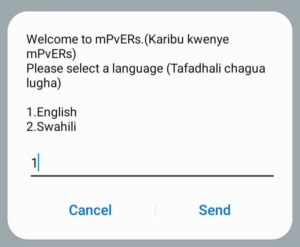
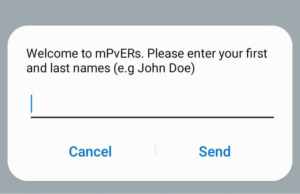
Below is a stepwise visual guide on registering on the platform.
Step 1: Dial *271# and choose a language. Step 2: Enter your names.
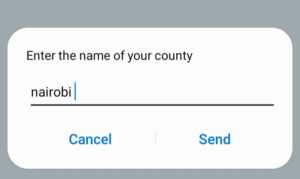
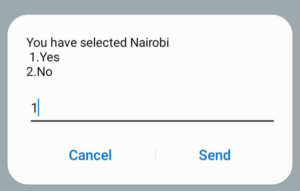
Step 3: Enter the name of the county you reside Step 4: Confirm the county
Step 5: Enter your email address.
Having completed the above steps, you will get registered on the platform and will be able to report any adverse reactions or poor-quality medication. The next time you dial *271#, the following interface will appear on your screen.


Once you have selected your preferred language, you can then proceed to report the adverse drug reaction by following the prompts provided in the same manner you did to get registered.
One of our primary duties as responsible citizens is healthcare safety. It’s better to work together in reporting any adverse drug reactions and poor quality medications so that the medicines every individual consumes are of optimum standard for developing a healthy nation.
“There’s no better policy in society than pursuing the health and safety of its people.” – Ralph Nader.
Written by:
Dr. Diana Sunday Migan,
Patient Safety Advocate.

Comments 1